What is qualification in the pharmaceutical industry? Qualification, of stability chambers to ICH guidelines is usually only required in the Pharmaceutical and Medical Devices industries and includes an IQOQPQ or IPV. What is an IQOQPQ? IQOQPQ stands for Installation Qualification, Operation Qualification, Performance Qualification. During installation of a stability chamber an IQOQPQ is often performed. […]
Tag Archives: qualification
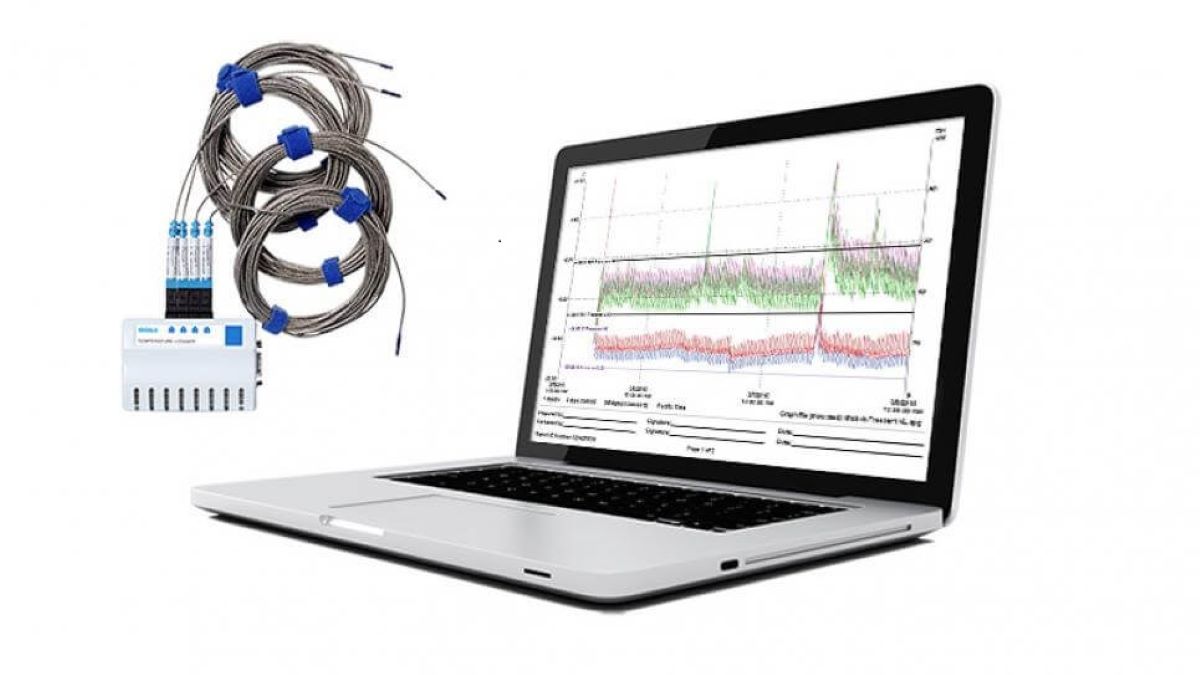
Introduction In Chamber Mapping and Qualification we describe how mapping is used in the Pharmaceutical and Medical Devices Industries and the mapping service DACTEC provides. Each market’s regulatory authority e.g., the FDA (Food & Drug Administration) in America, the HPRA (Health Products Regulatory Authority) in Ireland mandates the validation of environmental conditions that can affect […]